Upper GI
7. Oesophageal Cancer
Incidence and Epidemiology
- Prevalence: 7th most common cancer globally with 604,000 new cases in 2020.
- Mortality: 6th leading cause of cancer-related deaths (~544,000 in 2020).
- Demographics: Higher incidence in men (70% of diagnoses).
- Geographic Variation: Higher rates in Eastern Asia, Southern Africa, and Northern Europe.
- Subtypes:
- Squamous Cell Carcinoma (SCC): ~90% of global cases.
- Adenocarcinoma (AC): Increasing in North America and Europe due to lifestyle and dietary changes.
Risk Factors
- Adenocarcinoma (AC):
- Obesity, GERD, Barrett’s esophagus, positive family history, smoking, radiation therapy (RT), male gender, advanced age.
- Lower esophageal sphincter (LOS) relaxers: nitrates, anticholinergics, beta-agonists, aminophylline, benzodiazepines.
- Squamous Cell Carcinoma (SCC):
- Alcohol, smoking (synergistic effect), poor oral hygiene, socioeconomic deprivation.
- Nutritional deficiencies (zinc, selenium), palmoplantar keratoderma, and potentially HPV.
- Geographic-specific factors: betel quid chewing (India), hot beverage consumption (Iran, Tanzania).
Pathophysiology
- SCC: Arises from squamous epithelium.
- AC: Develops in the context of Barrett’s esophagus or GERD, often involving intestinal metaplasia.
Classification of Tumors
T-Stage Definitions
- T1a:
- M1: Carcinoma in situ.
- M2 (LPM): Invasion into the lamina propria.
- M3 (MM): Invasion into the muscularis mucosa.
- Management: Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) if >2cm.
- T1b:
- SM1: Invasion into the upper third of the submucosa.
- SM2: Invasion into the middle third of the submucosa.
- SM3: Invasion into the lower third of the submucosa.
- Management: Involvement of submucosa increases lymphatic spread risk; radical surgery required if M0.
- T2-T3: Tumor invades the muscularis propria (T2) or adventitia (T3).
- T4a: Involvement of pleura, pericardium, azygous vein, or diaphragm.
- T4b: Involves trachea/bronchi, vertebral body, or major vessels (e.g., aorta).
Diagnostic Approach
Key Investigations
|
Procedure |
Purpose |
|
Upper GI endoscopy |
Detect lesions, confirm previous findings, and obtain biopsy (≥6 samples). |
|
Histopathology |
Differentiate SCC vs. AC; assess for neuroendocrine/melanocytic tumors. |
|
Immunohistochemistry (IHC) |
PD-L1 testing for checkpoint inhibitor eligibility (e.g., TPS ≥1%, CPS ≥10). |
|
Endoscopic ultrasound (EUS) |
T/N staging, lymph node biopsies, and tumor layer invasion assessment. |
|
Bronchoscopy (with EUS) |
Check posterior tracheal involvement (upper two-thirds tumors). |
|
CT (thorax, abdomen, pelvis) |
Evaluate local invasion, nodal status, and distant metastasis. |
|
PET-CT |
Detect distant metastases if CT is negative. |
|
Laparoscopy |
Identify peritoneal metastases (OGJ tumors crossing diaphragm). |
Guidelines for Diagnosis:
- Initial Staging:
- CT Scan: Essential to evaluate for distant metastases.
- PET-CT: Used if CT is negative to confirm no metastases.
- EUS: Performed after CT for loco-regional staging.
- Less accurate for early mucosal disease staging.
- Recommended to stage T1 tumors or high-grade dysplasia via endoscopic resection for precise depth assessment.
- Special Considerations:
- Dilating tumors is not recommended unless necessary (e.g., coeliac lymph node FNA).
- Coordination with surgical teams is crucial to avoid complications.
Staging and Risk Assessment
- System Used: AJCC/UICC TNM 8th edition.
- Essential Components:
- Physical examination.
- FDG-PET: Preferred for detecting distant metastases.
- EUS: Recommended for evaluating T4b status and lymph node involvement.
- Laparoscopy: Used for peritoneal spread assessment in locally advanced OGJ tumors.
Nutritional and Functional Evaluation
- 50% of patients experience >5% body weight loss pre-surgery.
- Follow ESPEN guidelines for early nutritional support [II, A].
- Prehabilitation improves physical fitness and QoL [III, A].
- Geriatric assessment improves chemotherapy tolerance predictions [III, B].
Management of Localized and Locoregional Disease
Multidisciplinary Team (MDT) Assessment
- Treatment tailored to:
- Histology (SCC vs. AC).
- Clinical TNM stage.
- Tumor location.
- Performance status.
Early Disease (T1 N0 M0)
- Endoscopic Resection (preferred for T1 tumors):
- Techniques: EMR or ESD.
- Indications: High-grade dysplasia or T1a tumors without lymph node metastasis.
- Factors for Additional Treatment: Submucosal invasion (T1b+), poor differentiation, lymphovascular invasion.
Locally Advanced Disease (T2-T4, N+ M0)
- Surgery:
- Radical transthoracic esophagectomy with two-field lymphadenectomy.
- Minimally Invasive Esophagectomy (MIO): Shown to reduce morbidity and improve QoL.
Neoadjuvant/Perioperative Therapy
|
Histology |
Recommended Regimen |
Evidence Level |
|
SCC |
Preoperative CRT (CROSS regimen) |
I, A |
|
AC |
Perioperative FLOT chemotherapy |
I, A |
|
SCC (cervical) |
Definitive CRT (organ preservation) |
III, B |
Adjuvant Nivolumab:
- Indicated for residual disease after neoadjuvant CRT (CheckMate 577).
Definitive Chemoradiotherapy (CRT)
- Standard Regimen: Cisplatin + 5-FU or carboplatin-paclitaxel with 50.4 Gy radiation.
- Considerations:
- RT dose escalation >50.4 Gy not recommended due to increased toxicity.
- Salvage Esophagectomy: Performed for persistent disease [II, B].
Management of Advanced/Metastatic Disease
First-Line Therapy
- Standard Chemotherapy: Platinum-fluoropyrimidine doublet [II, A].
- Combination with ICIs (Checkpoint Inhibitors):
- Pembrolizumab + Chemotherapy (KEYNOTE-590).
- Nivolumab + Chemotherapy (CheckMate 648).
PD-L1 Subgroup Recommendations:
|
PD-L1 CPS |
Treatment |
Grade |
|
≥10 |
Pembrolizumab-Cisplatin/5-FU |
I, A |
|
≥1% (TPS) |
Nivolumab-Cisplatin/5-FU |
I, A |
Second-Line Therapy
- Nivolumab monotherapy (ATTRACTION-3 trial).
- Pembrolizumab for CPS ≥10 if ICI-naïve.
- Chemotherapy: Taxane or irinotecan for fit patients [II, B].
Supportive Care
- Early referral to palliative care.
- Nutritional intervention following ESPEN guidelines.
- Endoscopic stenting for palliation (in advanced disease).
Follow-Up and Survivorship
Surveillance
- Majority (~90%) of relapses occur within 2 years.
- Endoscopy, biopsies, and CT scans recommended every 3 months post-CRT for early recurrence.
Long-Term Care
- Address nutrition, psychosocial needs, and rehabilitation.
- Monitor for metachronous malignancies.
References
- ESMO Clinical Practice Guidelines
- Annals of Oncology (Obermannová et al., 2022)
- KEYNOTE and CheckMate studies for immunotherapy efficacy.
Images examples:
Figure 1. Oesophageal Stricture
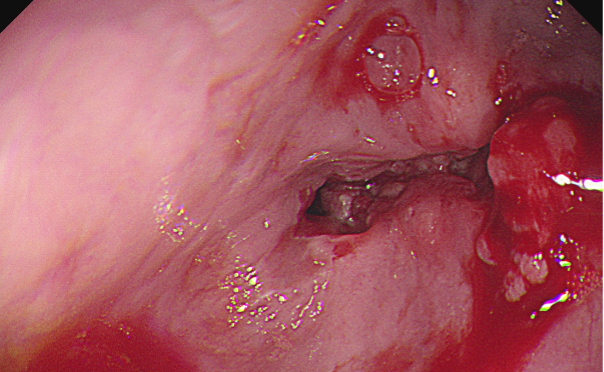
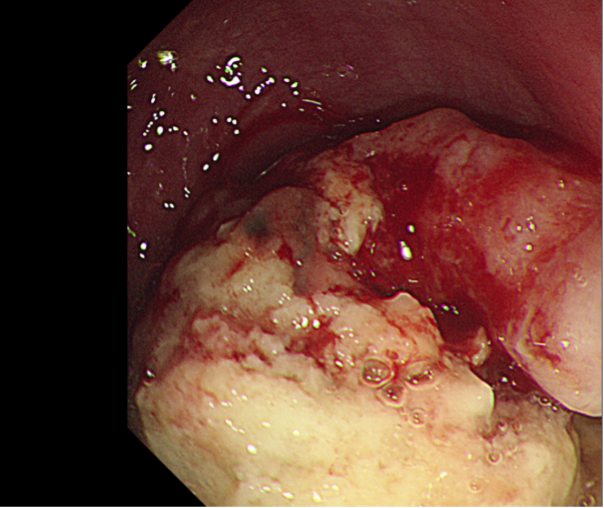
Figure 3. Oesophageal Pappiloma
