Upper GI
Completion requirements
5. Neuroendocrine Tumors
1. Gastrointestinal Neuroendocrine Tumors (GI NETs)
Overview
- GI NETs are neoplasms derived from neuroendocrine cells and classified by location and clinical context.
1.1 Gastric NETs
Subtypes of Gastric NETs
- Type 1 (70–80%):
- Associated Conditions: Chronic atrophic gastritis, pernicious anemia.
- Pathophysiology:
- Gastric achlorhydria → elevated serum gastrin → neuroendocrine cell hyperplasia → multifocal polypoid NETs.
- Clinical Features:
- Indolent course, low metastatic potential.
- Grade 1, Stage I tumors.
- Management:
- Endoscopic resection (ER) for tumors <2 cm.
- Endoscopic surveillance every 6–12 months.
- Type 2 (5%):
- Associated Conditions: Zollinger-Ellison Syndrome (ZES) from gastrinoma.
- Pathophysiology: Hypergastrinemia leads to NET formation.
- Clinical Features:
- Intermediate malignant potential.
- Often multifocal but less aggressive than sporadic NETs.
- Management:
- Endoscopic resection for tumors <2 cm.
- Surveillance every 6–12 months.
- Type 3 (Sporadic, 15–20%):
- Associated Conditions: None.
- Pathophysiology: Not related to elevated gastrin.
- Clinical Features:
- Aggressive with high metastatic potential (local and hepatic metastases in up to 65%).
- Management:
- Partial or total gastrectomy with lymphadenectomy.
- Systemic therapy for metastatic disease.
Diagnostic Approach
- Endoscopy with Biopsy: Diagnostic gold standard.
- Serum Markers:
- Chromogranin A (elevated in most NETs).
- Serum gastrin (especially elevated in Type 1 and 2).
- Imaging:
- CT/MRI for staging.
- Ga68-PET/CT for somatostatin receptor imaging.
Prognosis
- Type 1 NETs: Excellent prognosis.
- Type 3 NETs: Poor prognosis due to aggressive nature.
1.2 Colorectal Neuroendocrine Tumors (NETs)
Overview
- Colon NETs: Rare but aggressive.
- Rectal NETs: More common and generally indolent.
Incidence
- Colorectal NETs constitute ~1% of all colorectal tumors.
- Rectal NETs: Often detected due to increased colonoscopy screening.
Clinical Features
- Rectal NETs:
- Small (<1 cm), low-grade, and often asymptomatic.
- Larger tumors may present with rectal bleeding or pain.
- Colon NETs:
- Frequently present with bowel obstruction or distant metastases.
Risk of Metastases
- Rectal NETs:
- Tumors <1 cm: <5% risk.
- Tumors 1–2 cm: Intermediate risk (~10–15%).
- Tumors >2 cm: High risk (>60%).
- Colon NETs: Higher metastatic potential regardless of size.
Management
- Rectal NETs:
- <1 cm: Endoscopic mucosal resection (EMR).
- 1–2 cm: Endoscopic submucosal dissection (ESD) or transanal excision.
- >2 cm or invasive features: Surgical resection with lymphadenectomy.
- Colon NETs:
- Segmental colectomy with lymph node dissection.
Follow-Up Recommendations
- Low-risk rectal NETs (<1 cm): Endoscopic surveillance at 12 months.
- Higher-risk tumors: Surveillance every 6–12 months with imaging and colonoscopy.
1.3 Pancreatic Neuroendocrine Tumors (PanNETs)
Overview
- Incidence: ~1 case per 100,000 annually.
- Types:
- Functional: Hormone-producing (e.g., insulinomas, gastrinomas).
- Non-functional: No hormone secretion (more common).
Clinical Features
- Symptoms depend on hormonal secretion:
- Insulinoma: Hypoglycemia.
- Gastrinoma (ZES): Peptic ulcers, diarrhea.
Indications for Surgical Resection
- Tumor Size:
- 2 cm: Surgery recommended.
- <2 cm: Consider observation or surgery based on functional status, symptoms, and growth.
- Other Criteria:
- Presence of metastases.
- Functional tumors with significant hormone secretion.
- Tumors causing symptoms due to local invasion.
Management
- Localized Disease:
- Surgical resection (e.g., enucleation for small tumors, distal pancreatectomy for larger tumors).
- Hormonal Therapy:
- Somatostatin analogs (e.g., octreotide) for hormone-related symptoms.
- Considered in metastatic or inoperable cases to control symptoms.
- Metastatic Disease:
- Targeted therapies (e.g., everolimus, sunitinib).
- Peptide receptor radionuclide therapy (PRRT) for advanced cases.
2. Gastrointestinal Stromal Tumors (GISTs)
Overview
- GISTs are mesenchymal tumors originating from interstitial cells of Cajal.
- Incidence: 7–15 cases per million annually.
- Most Common Location: Stomach (~60%).
Pathophysiology
- Caused by activating mutations in KIT (CD117) or PDGFRA genes.
- Familial GISTs (5%): Associated with conditions such as:
- Neurofibromatosis.
- Carney-Stratakis syndrome (paraganglioma + GIST).
Histological and Diagnostic Features
- Histology:
- Spindle cell, epithelioid, or mixed morphology.
- CD117 (KIT) and DOG-1 positive.
- Imaging:
- CT: Large, smooth submucosal mass.
- EUS: Subepithelial lesion arising from the muscularis propria.
- PET-CT: Useful for metastatic assessment.
Prognostic Factors
- Tumor size (>5 cm) and high mitotic rate correlate with worse outcomes.
- Poor prognosis associated with mesenteric fat infiltration, ulceration, and lymphadenopathy.
Clinical Presentation
- Symptoms:
- GI bleeding (28% for small intestine, 50% for gastric).
- Abdominal pain: 8–17%.
- Incidental asymptomatic mass: 13–18%.
- Acute abdomen: 2–14%.
Management
- Surgical Resection:
- Indicated for tumors >2 cm.
- Goal: R0 resection (negative margins).
- Adjuvant and Neoadjuvant Therapy:
- Imatinib (Tyrosine Kinase Inhibitor): For high-risk or large tumors.
- Sunitinib or regorafenib: For imatinib-resistant cases.
- Metastatic Disease:
- Systemic therapy with TKIs.
- Consider surgery for isolated metastases.
Prognosis
- 5-year survival: ~70–80% for localized gastric GISTs post-resection.
- Poorer prognosis for tumors >10 cm or metastatic disease.
References
- European Society for Medical Oncology (ESMO) Guidelines.
- British Society of Gastroenterology (BSG) Guidelines.
- National Comprehensive Cancer Network (NCCN) Guidelines.
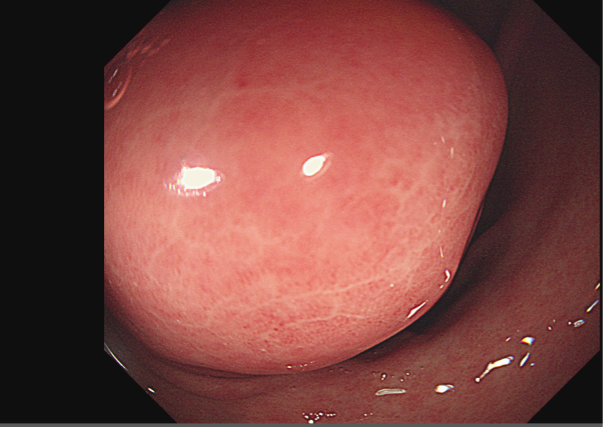
Figure 1: GIST.