Upper GI
Completion requirements
2. Gastric Cancer
1. Epidemiology and Incidence
- Global Distribution:
- High Incidence: Eastern Asia (Japan, South Korea, China), Andean regions of South America, and Eastern Europe.
- Low Incidence: North America, Northern Europe, Africa, Southeast Asia (including India).
- UK Regional Trends:
- Higher incidence in Northern regions compared to Southern regions.
- Demographics:
- Peak age: 50–60 years.
- Male-to-female ratio: 2–12:1.
- Trends:
- Decline in distal (non-cardia) gastric cancers.
- Increase in proximal (cardia) gastric cancers.
2. Pathophysiology and Risk Factors
- Carcinogenesis:
- Chronic inflammation leading to intestinal metaplasia and dysplasia.
- Key pathways: Helicobacter pylori infection, genetic mutations (e.g., CDH1 for hereditary diffuse gastric cancer).
- Key Risk Factors:
- H. pylori infection (most important risk factor).
- High-salt diet, processed meats, cured fish.
- Smoking and obesity.
- Prior gastric surgery (e.g., Billroth II).
- Genetic predisposition: hereditary conditions (FAP, Lynch syndrome).
- Protective Factors:
- Diet rich in fruits and vegetables.
- Use of NSAIDs (may reduce risk).
3. Clinical Presentation
- Symptoms of Early-Stage Disease: Often asymptomatic.
- Symptoms of Advanced-Stage Disease:
- Weight loss, epigastric pain, early satiety.
- Nausea, vomiting.
- Dysphagia (if proximal tumor).
- Occult GI bleeding → anemia.
- Virchow’s node (left supraclavicular lymphadenopathy).
4. Diagnostic Approach
Key Investigations
- Endoscopy with Biopsy:
- Gold standard for diagnosis.
- Obtain ≥6 biopsy samples from the lesion margins and central regions.
- Endoscopic Ultrasound (EUS):
- Evaluates depth of tumor invasion (T stage) and lymph node involvement (N stage).
- Imaging for Staging:
- CT Scan (Chest, Abdomen, Pelvis): Rules out distant metastases.
- PET-CT: Used when CT findings are ambiguous.
- Laparoscopy: Assesses for peritoneal and hepatic metastases, especially for locally advanced disease.
5. Staging and Classification
- AJCC/UICC TNM Staging:
|
T Stage |
Description |
|
T1 |
Invades lamina propria, muscularis mucosae, or submucosa. |
|
T2 |
Invades muscularis propria. |
|
T3 |
Invades subserosa. |
|
T4 |
Invades serosa or adjacent structures. |
- N Stage: Number of regional lymph nodes involved.
- M Stage: Presence of distant metastases.
6. Management
Early-Stage Disease (T1, No Lymph Node Involvement)
- Endoscopic Resection:
- Endoscopic Mucosal Resection (EMR) or Endoscopic Submucosal Dissection (ESD):
- Indications: Lesions <2 cm, confined to mucosa, with no ulceration and low lymph node metastasis risk.
- EMR for lesions <1 cm; ESD for larger lesions or those with superficial submucosal invasion.
Locally Advanced Disease (T2–T4, N+)
- Surgery:
- Subtotal Gastrectomy for distal tumors.
- Total Gastrectomy for proximal tumors.
- Transhiatal Total Gastrectomy for GOJ (Type II) tumors.
- D2 lymphadenectomy (removal of ≥15 lymph nodes).
- Perioperative Chemotherapy:
- FLOT regimen (5-FU, leucovorin, oxaliplatin, docetaxel): Standard for resectable tumors.
- Alternative: Epirubicin, cisplatin, and capecitabine (ECX) for selected patients.
Metastatic Disease (M1)
- Systemic Chemotherapy:
- Platinum-fluoropyrimidine doublet (e.g., cisplatin/5-FU).
- Addition of trastuzumab for HER2-positive tumors.
- Second-line options: Ramucirumab (VEGF receptor 2 inhibitor), paclitaxel.
- Immunotherapy:
- PD-1 inhibitors (nivolumab, pembrolizumab) for patients with PD-L1-positive tumors.
7. Surveillance and Follow-Up
- Post-Treatment Surveillance:
- Clinical assessments every 3–6 months for the first 2 years.
- Imaging (CT, PET-CT) and endoscopy as needed.
- ESMO Guidelines:
- Routine follow-up with imaging in high-risk patients.
- Symptom-based follow-up for low-risk patients.
- Screening and Prevention:
- Eradication of H. pylori reduces gastric cancer risk.
- Consider genetic testing for patients with a family history of hereditary diffuse gastric cancer.
References
- British Society of Gastroenterology (BSG) Guidelines.
- European Society for Medical Oncology (ESMO) Guidelines.
- Annals of Oncology Review on Gastric Cancer.
Figure 1: GI lymphoma.
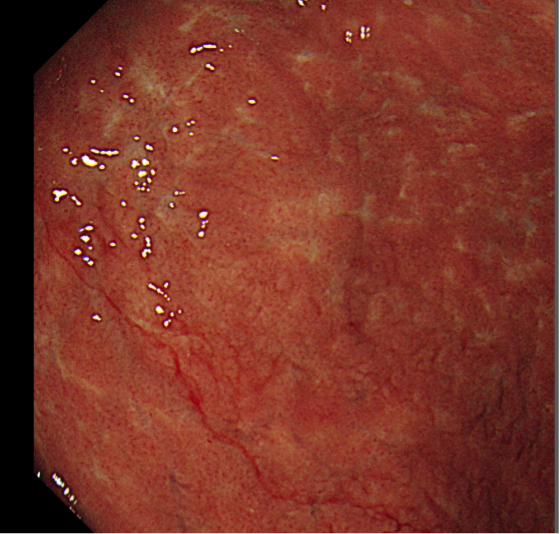
Figure 2: Malignant gastric stricture.
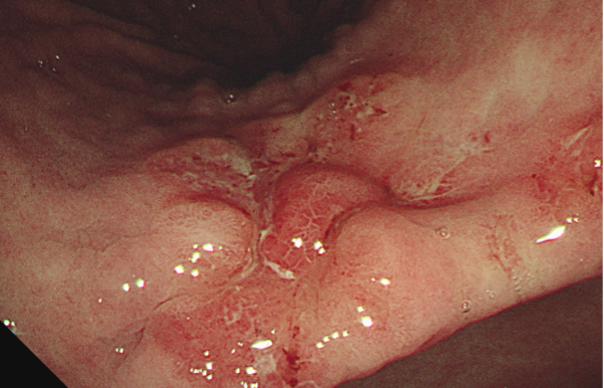
Figure 3: Malignant gastric ulcer.
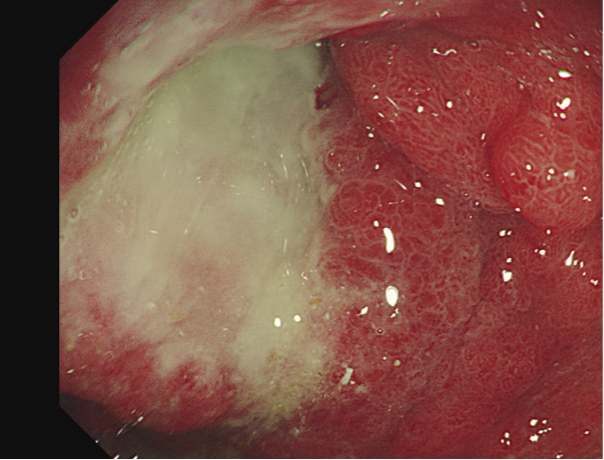
Figure 4: linitis with acute GI bleeding.
